Tianeptine, known as “Gas Station Heroin,” Joins the Ranks of Illegal Drugs Harming Americans
As recently as January 23, 2024, the U.S. Food and Drug Administration (“FDA”) reiterated a previous public health advisory to consumers: do not purchase or use any product containing the illegal and potentially dangerous substance tianeptine. Products containing tianeptine are known colloquially as “gas station heroin” because they: (1) are readily available in gas stations, as well as in smoke shops, convenience stores, and even online; and (2) purportedly produce an opioid-like euphoria in users, especially in high doses. Yet, tianeptine, despite its broad availability, is a potentially dangerous substance not FDA-approved for any medical use.
What Is Tianeptine?
Tianeptine was first developed as an antidepressant in the 1980s but is molecularly and mechanistically unique from other antidepressants and, therefore, is classified as an atypical tricyclic antidepressant. Although approved in low doses for treating depression in some countries, it has not been approved for use in any form or dosage in the United States. Nevertheless, tianeptine continues to be marketed broadly as a dietary supplement. Vendors sell it in powder, capsule, or liquid form, claiming its use improves brain function and treats anxiety, depression, pain, opioid use disorder, and other conditions.
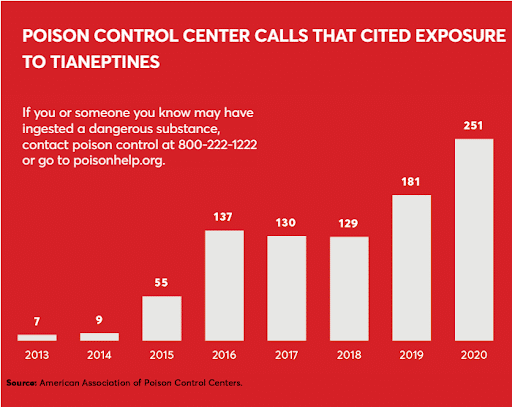
In November 2018, the FDA first warned of serious adverse events associated with tianeptine, noting in particular the danger of addiction. In issuing its warning, the FDA stated in no uncertain terms that tianeptine does not qualify as a dietary supplement and is considered an adulterated drug that is illegal to sell or distribute. Yet, sales of tianeptine have continued, and tianeptine-related calls to poison control centers have skyrocketed. Between 2015 and 2021, there have been at least 883 calls to poison control centers, up from 27 the decade before. At least four people have died after using the drug, and almost two dozen have reported hospitalization and other adverse effects.
The FDA's Warnings on Tianeptine
The FDA continues to receive severe adverse event reports concerning tianeptine products and has identified cases in which consumers experienced serious harmful effects from abusing or misusing tianeptine by itself or with other drugs, including antidepressants and anti-anxiety medicines. These effects included agitation, drowsiness, confusion, sweating, rapid heartbeat, high blood pressure, nausea, vomiting, slowed or stopped breathing, coma, and death.
Tianeptine is just the most recent newsworthy ingredient illegally sold as a dietary supplement, joining substances like kratom, that gained popularity among consumers seeking an opioid-like effect in a product that they assume is safe because it’s sold in everyday shopping outlets.
While the FDA has continued to issue warnings to numerous companies to cease selling tianeptine, addressing the illegal sale of this product has proven difficult. An FDA spokesperson says the agency has “no systematic way of knowing what dietary supplement products are on the market” or what ingredients they contain. “Because we cannot put an investigator in every store in every state, and we do not have a constantly updating window into every retail corner of the internet, stakeholders become our eyes and ears, and we are left trying to play catch-up,” she says.
Even when the FDA becomes aware of an outlet, its enforcement actions have not been sufficient to deter the flow of tianeptine products to consumers. Warning letters to retailers – one of the strongest tools available to the agency – are viewed by some recipients like traffic tickets, and the FDA doesn’t always follow up. The agency also issues import alerts, stopping products that are known to contain tianeptine at the border, though this method doesn’t impact products that are not yet known to the FDA.
Restricting & Banning Tianeptine On A State Level
On the local level, at least nine states have restricted or banned the ingredient, even labeling it a controlled substance in some states. A quick online search, however, turns up numerous retailers still peddling tianeptine products.
The danger posed by the continued sale of tianeptine and the difficulties encountered by the FDA in curbing its use has attracted the attention of Congress. On January 20, 2024, five members of the U.S. House of Representatives requested the FDA to review the substance and take more significant action. Moreover, on January 25, 2024, two U.S. Representatives announced bipartisan legislation — the Scheduling Tianeptine and Analogues Now to Defend Against Emerging Opioids Act (“STAND Against Emerging Opioids Act”) — that would classify tianeptine as a Schedule III controlled substance under the Controlled Substance Act. Should the STAND Against Emerging Opioids Act be enacted, sales of tianeptine would be illegal unless prescribed to prevent misuse and abuse, much like codeine and ketamine.
Potential changes within the FDA, and possibly new laws or regulations on the federal level, may impact the future of dietary supplements and the availability of substances like tianeptine. Until such changes are implemented, the FDA will need to continue to use the tools at its disposal to make the public aware of the dangers of products that it cannot completely keep off the shelves of gas stations and other retail outlets or prohibit from sale on the Internet.
Authored by Lisa Krist, VP, Chief Customer Focus Officer