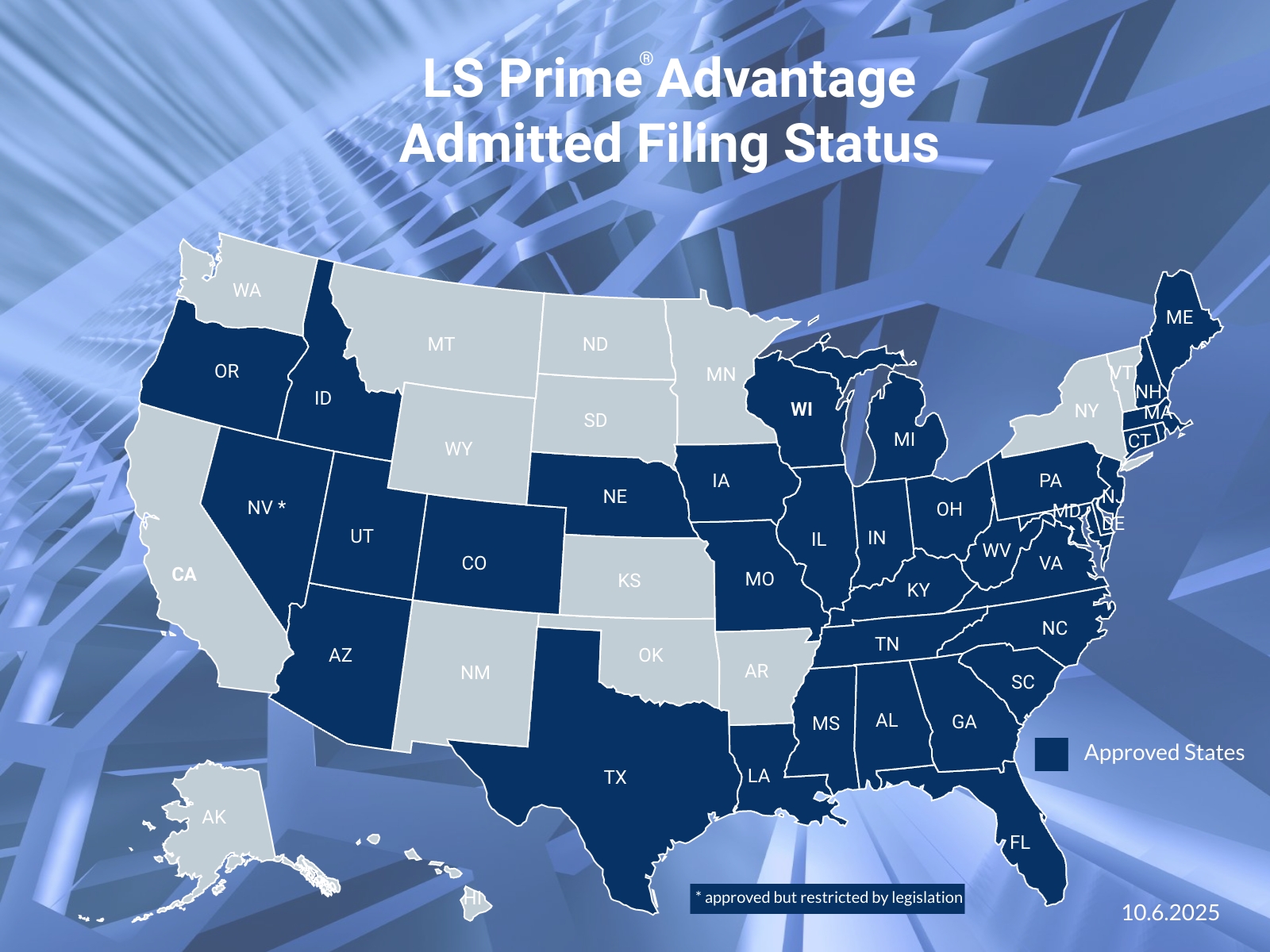
Tailored Life Sciences Insurance
Through appointed retail and wholesale brokers, our industry specialists offer comprehensive life sciences insurance solutions globally.
Feature descriptions are summaries for discussion purposes only and do not modify any policy.
Coverage is determined by the policy terms, conditions, and exclusions as issued and applicable law.


The Latest in Life Science

FDA’s 2026 Guidance Expands Pathway for Low-Risk Digital Health Products—But Caution Remains Essential
The new year has brought notable changes on the regulatory front for life science companies seeking to expand their portfolio of digital health products. In January 2026, the U.S. Food & Drug Administration (FDA) published updated guidance documents addressing two broad product categories: (1) low-risk digital health devices for general wellness use; and (2) clinical decision support (CDS) software.

GLP-1 Compounding: When Innovation May Mean Liability
As previously reported on our blog, the rise of compounded GLP-1 medications has sparked both excitement and concern across the healthcare and legal landscapes. As demand for weight-loss treatments increases, some pharmacies have begun offering compounded versions of popular drugs like semaglutide and tirzepatide.
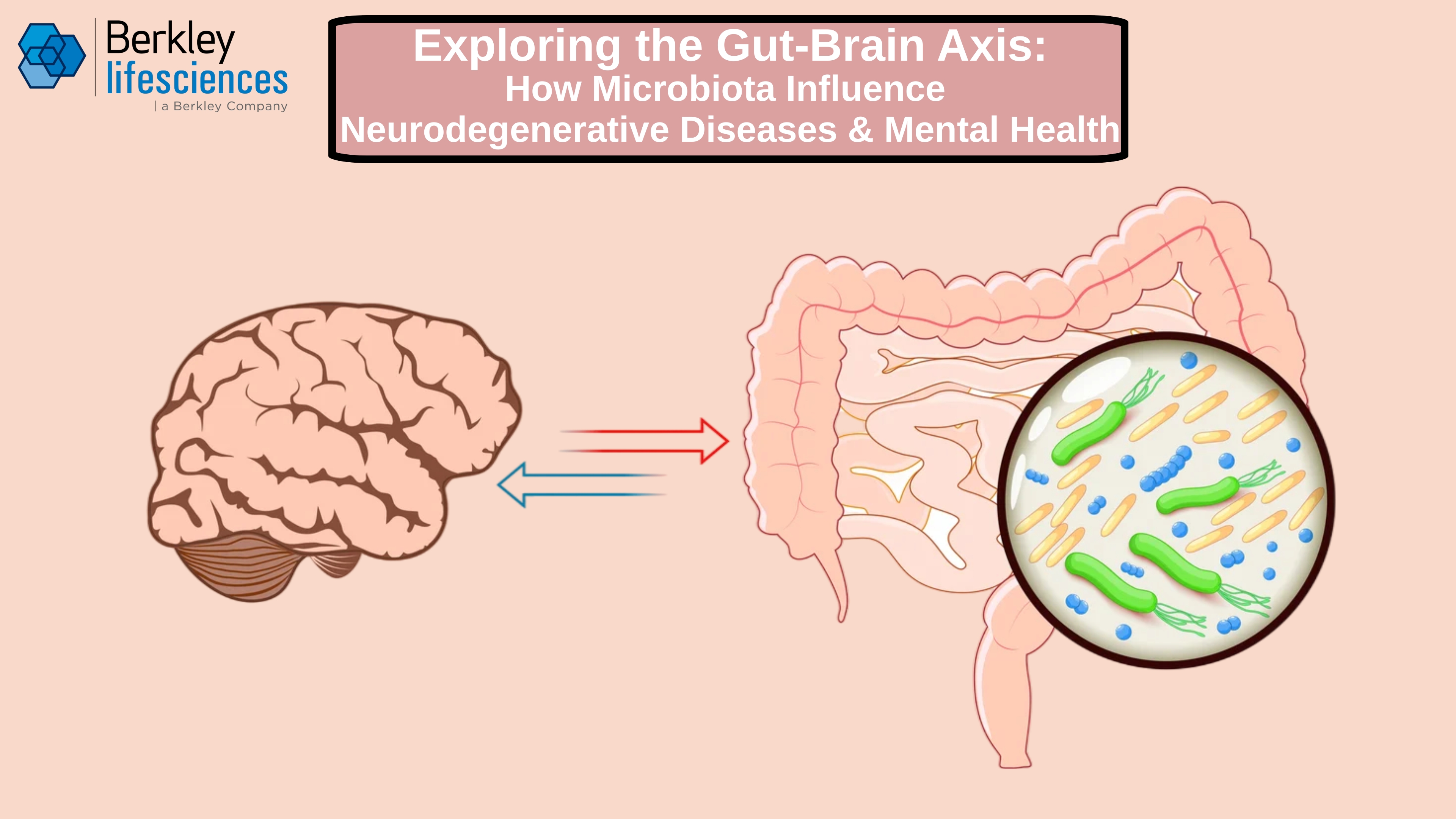
Exploring the Gut-Brain Axis: How Microbiota Influence Neurodegenerative Diseases and Mental Health
Researchers and physicians have historically taken a compartmentalized approach to medicine: neurologists treat conditions of the brain, gastroenterologists address concerns of the digestive system, and psychiatrists administer care for mental health. Seldom have these specialties intersected in meaningful ways.